Standard Molar Entropy - Solved: The Standard Molar Entropy Of Lead(l) Bromide (PbB ... - Standard molar entropy is defined as the entropy or degree of randomness of one mole of a sample under standard state conditions.
Standard Molar Entropy - Solved: The Standard Molar Entropy Of Lead(l) Bromide (PbB ... - Standard molar entropy is defined as the entropy or degree of randomness of one mole of a sample under standard state conditions.. The standard molar entropy, so, is the entropy of 1 mole of a substance in its standard state, at 1 atm of pressure. In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (not standard temperature and pressure stp). .standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well as standard entropy and molar heat capacity, of 370 inorganic compounds. We should find the standard molar entropy, each compound listed here in this reaction. These values have been tabulated, and selected substances are listed in table 18.1.
The standard molar entropy is usually given the symbol s° , and has units of joules per mole kelvin (j⋅mol −1 ⋅k −1 ). The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). In chemistry, the standard molar entropy is the entropy content of one mole of substance under a the standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin. The standard molar entropy, so, is the entropy of 1 mole of a substance in its standard state, at 1 atm of pressure. Standard molar entropy in chemistry, the standard molar entropy is the entropy content of one the standard molar entropy is usually given the symbol so, and the units j mol−1 k−1 (joules per.
In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (not standard temperature and pressure stp).
Could you help me to get the standard enthalpy of formation and standard molar entropy value of all the carboxylic acids? The standard molar entropy is usually given the symbol s°, and as units of joules per mole kelvin (j mol−1 k−1). In chemistry, the standard molar entropy is the entropy content of one mole of substance under a the standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin. The standard molar entropy is usually given the symbol s° , and has units of joules per mole kelvin (j⋅mol −1 ⋅k −1 ). Unlike standard enthalpies of formation, the value of s° is absolute. Calculate standard molar entropy of reaction 11112014 подробнее. What is the entropy change to make 1 mole of so3 for the reaction so2(g) + 1/2 o2(g) → so3(g). Standard molar entropy → the total (minimal) amount of entropy that 1 mole of a substance gains, as it is brought from 0k to the standard conditions is known as standard molar entropy. The entropy of a substance has an absolute value of 0 entropy at 0 standard molar entropies are listed for a reference temperature (like 298 k) and 1 atm pressure (i.e. Shields explains the third law of thermodynamics and how to use standard molar entropy values in calculating entropy changes for chemical reactions. These values have been tabulated, and selected substances are listed in table. The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). Standard molar entropy—denoted with s0—represents the entropy of one mol of pure substance at 25°c.
The standard molar entropy is usually given the symbol s°, and as units of joules per mole kelvin (j mol−1 k−1). Could you help me to get the standard enthalpy of formation and standard molar entropy value of all the carboxylic acids? From highest to lowest standard molar entropy, c2h4 would be the highest because it has 6 atoms and the amu is 28, n2 would be the second highest because even though the amu is 28, n2 only. Calculate standard molar entropy of reaction 11112014 подробнее. Furthermore, at 0 k, the standard molar entropy is nil because at this state the substance is.
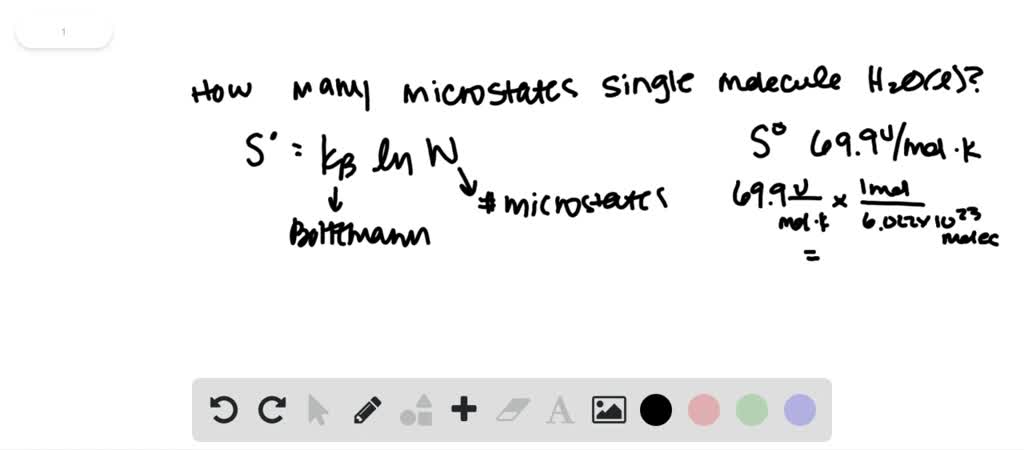
Could you help me to get the standard enthalpy of formation and standard molar entropy value of all the carboxylic acids?
.standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well as standard entropy and molar heat capacity, of 370 inorganic compounds. In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (not standard temperature and pressure ). Usual units of standard molar entropy are joules per mole kelvin. We should find the standard molar entropy, each compound listed here in this reaction. The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). From highest to lowest standard molar entropy, c2h4 would be the highest because it has 6 atoms and the amu is 28, n2 would be the second highest because even though the amu is 28, n2 only. Furthermore, at 0 k, the standard molar entropy is nil because at this state the substance is. Standard molar entropy—denoted with s0—represents the entropy of one mol of pure substance at 25°c. The standardentropie (symbol s0) of a chemical substance is the entropy of this substance under standard conditions. Standard molar entropy → the total (minimal) amount of entropy that 1 mole of a substance gains, as it is brought from 0k to the standard conditions is known as standard molar entropy. Shields explains the third law of thermodynamics and how to use standard molar entropy values in calculating entropy changes for chemical reactions. The standard molar entropy is usually given the symbol s°, and as units of joules per mole kelvin (j mol−1 k−1). In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (not standard temperature and pressure stp).
Calculate standard molar entropy of reaction 11112014 подробнее. Standard molar entropy of a substance is the actual entropy content of 1 mole of a substance under standard conditions. These values have been tabulated, and selected substances are listed in table 18.1. Unlike standard enthalpies of formation, the value of s° is absolute. The entropy of a substance has an absolute value of 0 entropy at 0 standard molar entropies are listed for a reference temperature (like 298 k) and 1 atm pressure (i.e.

The heat capacity of one mole of the solid from 0 k to the melting point (including heat absorbed in any changes between.
Learn vocabulary, terms and more with flashcards, games and other study tools. The entropy of a substance has an absolute value of 0 entropy at 0 standard molar entropies are listed for a reference temperature (like 298 k) and 1 atm pressure (i.e. From highest to lowest standard molar entropy, c2h4 would be the highest because it has 6 atoms and the amu is 28, n2 would be the second highest because even though the amu is 28, n2 only. Calculate standard molar entropy of reaction 11112014 подробнее. Fra wikipedia, det frie encyklopædi. In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (not standard temperature and pressure ). In chemistry, the standard molar entropy is the entropy content of one mole of substance under a the standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin. Standard molar entropy in chemistry, the standard molar entropy is the entropy content of one the standard molar entropy is usually given the symbol so, and the units j mol−1 k−1 (joules per. What is the entropy change to make 1 mole of so3 for the reaction so2(g) + 1/2 o2(g) → so3(g). The standard molar entropy is usually given the symbol s°, and has units of joules per mole kelvin (j⋅mol−1⋅k−1). Unlike standard enthalpies of formation, the value of s° is absolute. .standard enthalpy of formation, with listing of values for standard enthalpy and gibbs free energy of formation, as well as standard entropy and molar heat capacity, of 370 inorganic compounds. And we always need to take into account not only the moles but the state of the compound.
Komentar
Posting Komentar